valence electrons co2|Iba pa : Baguio In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical prope. You know i had to do it to em, the meme that took over the world over the last 3 years, it doens't seem to die out, it kee. Alright, you know what's up right!
PH0 · valence electrons worksheet
PH1 · valence electrons in o2
PH2 · valence electrons chart
PH3 · how to calculate valence electrons
PH4 · how many valence electrons nitrogen
PH5 · how many valence electrons does co2 have
PH6 · how many valence electrons are in co2
PH7 · co2 valence electron number
PH8 · Iba pa
Assista vídeos pornô de Neymar de graça, aqui no Pornhub.com. Descubra a crescente coleção de vídeos e filmes Mais relevantes explícitos em alta qualidade. Nenhum outro site pornô é mais popular e tem mais cenas de Neymar do que o Pornhub! Navegue pela nossa incrível seleção de de vídeos pornô em HD em qualquer dispositivo que você tenha em .
valence electrons co2*******To determine the number of valence electrons for CO2, the Carbon dioxide molecule, we’ll use the Periodic Table. You may assume the valences of the chemical elements—the number of .In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical prope.The presence of a sigma bond and valence electron pairs repelling each other force them to move to the opposite side of the carbon atom, resulting in this geometric shape. As a result, the carbon atom takes .
The valence electrons of carbon dioxide are the sum of the total valence electrons of carbon and oxygen in the compound CO 2. The carbon dioxide compound has a total of sixteen electrons in the last .There are oxygen and carbon as elements in carbon dioxide. Oxygen belongs to the group VIA and contains six electrons in its last shell. Carbon belongs to the group IVA and . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s .An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons .
Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. These valence electrons are represented by drawing dots around the individual .
Carbon atoms are always central; b) Connect the central atom with each of the terminal atom by drawing a single bond. 3. For each single bond, subtract two electrons from the total number of valence .
Iba paCarbon is a member of the IVA group and has four electrons in its valence shell. Therefore, the total number of valence electrons required to draw the Lewis structure of CO 2 = 6(2) + 4 = 16. 2. Total electron . Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition .
To determine the valence electrons of CO 2, it is first necessary to know the valence electrons of the oxygen and carbon atoms. To determine the valence electrons of carbon dioxide we have to follow two steps. It is shown below: Step 1: Determine the valence electrons of carbon and oxygen atoms. The atomic number of .A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. . In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
Step #1: Calculate the total number of valence electrons. Here, the given molecule is CO2 (carbon dioxide). In order to draw the lewis structure of CO2, first of all you have to find the total number of valence electrons present in the CO2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an . For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. We can use this method to predict the charges of ions in ionic .
Magnesium has two valence electrons. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. The highest principal quantum number is 2. There are 2 electrons in the 2s subshell and 2 electrons in the 2 p subshell, giving carbon a total of four valence electrons. Bromine’s ground state electron configuration is 1s 2 2s 2 p 6 .

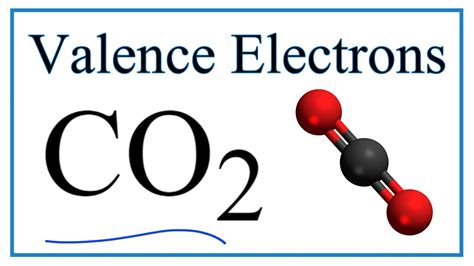
CO2 has a total of 16 valence electrons (carbon has 4 and oxygen 6 valence electrons). CO2 has a linear molecular geometry with a bond angle of 180° on a plan. Molar mass of CO2 is 44.01 g/mol which is also known as molecular weight. Carbon dioxide has an sp hybridization type because the steric number of central carbon is 2.
4.E: Valence Electrons and Bonding (Exercises) These are homework exercises to accompany Chapter 4 of the Furman University's LibreText for CHE 101 - Chemistry and Global Awareness. Valence electrons are outer shell electrons with an atom and can participate in the formation of chemical bonds. In single covalent bonds, . For #CO_2# there are #4(C)+2xx6(O)# valence electrons...i.e. 8 electron pairs to distribute over three centres.. Explanation: .i.e. these are the #2s^2# and #2p^2# electrons of carbon, and the #2s^2# and #2xx2p^2# of oxygen..16 electrons in total..So carbon also has 8 valence electrons. CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a .Carbon has 4 valence electrons, while each oxygen atom has 6 valence electrons. In total, CO2 has 16 valence electrons. Next, we need to arrange the atoms in the molecule. Carbon is the central atom in CO2, with each oxygen atom bonded to it by a double bond. A double bond consists of two pairs of electrons, one from each atom. The standard atomic mass of carbon is 12.0096 and its symbol is ‘C’. The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons in the last shell after the electron configuration of carbon is called the valence electrons of carbon. The last shell of carbon has four electrons.valence electrons co2The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. The reason for drawing/creating a Lewis dot structure is that it helps one predict the kinds of bonds, as well as a number of bonds, that can be formed around an atom. Lewis structures can be utilized to make . In our example, since carbon is in group 14, we can say that one atom of carbon has four valence electrons. Advertisement. Transition Metals 1. Find an element from Groups 3 to 12. As noted above, the elements in groups 3 to 12 are called "transition metals" and behave differently than the rest of the elements when it comes to valence . Looking at the 2s 2 2p 2 valence electron configuration of carbon, we might expect carbon to use its two unpaired 2p electrons to form compounds with only two covalent bonds. We know, however, that carbon typically forms compounds with four covalent bonds. We can explain this apparent discrepancy by the hybridization of the 2s .
All odds as of Sunday morning. Spread: 49ers (-2) Moneyline: 49ers (-130), Chiefs (+110) Over/under: 47.5 Flag football:Pro Bowl highlights growing sport that welcomes all We occasionally .
valence electrons co2|Iba pa